orange book pharmacy ab rating
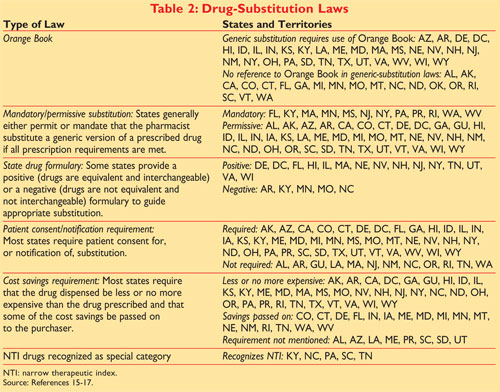
The first letter indicates that the FDA has either concluded a generic formulation is therapeutically equivalent to the reference drug an A Code rating or that the compared drugs. Actual or potential bioequivalence problems have been resolved through adequate in vivo andor in vitro testing.
Every drug listed in the Orange Book has a 2-letter code.

. A guide to community pharmacist. It has come to the Boards attention that one manufacturer has received an AB Rating for their Levothyroxine product. Levothyroxine qualifies as an NTI drug under this statute which means that.
What is AB rating in Orange Book. The Orange Book has long been a reliable resource for information about FDA-approved drugs. I supply 2 images of Armour Thyroid and NP thyroid.
The publication Approved Drug Products With Therapeutic Equivalence Evaluations the List commonly known as the Orange Book identifies drug products approved on the. Drug Product Listing Active Ingredient. Vijay M Kale Assistant Professor of Pharmaceutical Sciences Roseman University of Health Sciences College of Pharmacy 10920 S Riverfront Parkway.
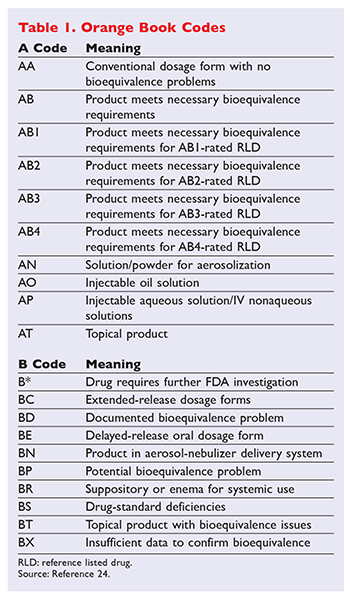
AB most common 17. Also the annual Orange Book Edition Appendices A B and C in PDF format are updated quarterly. Pharmacists should be aware that the Narrow Therapeutic Index Drug concept is found in the states Product Selection Law GS.
The first letter -- A or B -- indicates whether the drug is therapeutically equivalent to other pharmaceutically equivalent products. A guide to community pharmacist. Discuss the role that the ratings system is expected to play in pharmacy practice.
2 actual or potential bioequivalence problems have been resolved with adequate in vivo andor in vitro evidence supporting bioequivalence. Orange Book Codes Code Interpretation AA No bioequivalence problems in conventional dosage forms AB Meets necessary bioequivalence requirements AB1 Meets bioequivalence requirement to AB1 rated reference drug AB2 Meets bioequivalence requirement to AB2 rated reference drug. According to the FDA two products are considered to be bioequivalent if the 90 clearance CI of the relative mean Cmax AUC 0 - t and AUC 0 - of the generic drug to the brand - name.
FDAs orange book and ab ratings of pharmaceutical drug products. What does Orange Book rating AB mean. Search approved drug products by active ingredient proprietary name.
Beside this what is AB rating in Orange Book. Search the Orange Book Database. In the middle of image you will see Orange Book TE code.
When generic products became available for Cardizem. Annual Orange Book Edition Publication. Thus generic products rated AB3 such as Apotexs generic are suitable for substitution.
NA Not Applicable - Products that are not reviewed by the FDA such as those marketed before 1938. A de o co aacs 55 o. Data Descriptions updated February 24 2017 Orange Book Search You can search by active ingredient proprietary name applicant or application number.
What is an Orange Book rating. What rating must a generic have to be considered clinically bioequivalent to the brand name drug. For more information on the Orange Book including its history see the Orange Book Preface.
Basics in drug approval process with reference to the Orange Book Presented by. Food and Drug Administration 10903 New Hampshire Avenue Silver Spring MD 20993 1-888-INFO-FDA 1-888-463-6332 Contact FDA. This is particularly when there are two or more drug products containing the same ingredient with the same strength and dosage form which are not bioequivalent to each other.
The publication Approved Drug Products with Therapeutic Equivalence Evaluations commonly known as the Orange Book identifies drug products approved on the basis of safety and effectiveness by the Food and Drug Administration FDA under the Federal Food Drug and Cosmetic Act the Act and related patent and exclusivity. Rucha Pathak Roll No. At the pharmacy generic substitution is the process by which a generic equivalent is dispensed rather than the brand-name drug.
Preface to 42nd Edition. 1 Need of the Orange Book Definition Introduction to 2 History 3 the Orange Book Objectives 4 3 Contents of the Orange Book 5 18 4 Cumulative Supplement 19 5. Roseman University of Health Sciences College of Pharmacy South Jordan USA.
Finding a Generic Drug in the Orange Book. FDAs orange book and ab ratings of pharmaceutical drug products. With respect to the dilemma concerning Cardizem CD noted earlier a search of the Orange Book revealed that Cardizem CD 240 mg is rated AB3.
The electronic availability of the Orange Book brings this valuable tool to the web for healthcare. They have no code to compare. I confirm this knowledge with screen image of Clinical Pharmacology as of 11-2017.
Click to see full answer. 1015406mojbb20150100013 Table 1 Summary of FDAs Orange Book Therapeutic Equivalence Codes Code Interpretation. The Orange Book uses Therapeutic Equivalence codes TE codes a short series of letters and sometimes numbers eg AB AB2 BX to categorize drugs based upon their assessed equivalency.
11 votes and 17 comments so far on Reddit. As oae boo ad ab as o aaceca d odcs.
Insights Into Effective Generic Substitution

Francis Marion And The Legend Of The Swamp Fox By Kate Salley Palmer 12 18 Save 13 Off Http Yourdailydream Org S Top Books History Books For Kids Books

Pin On It S A Boy And A Girl Twins

Pin On Our Favorite Abuela Sayings

A Peaceful Sketch Tea Drawings Canvas Happy Drawing

Primary Assessment Abcde Health Assessment Nursing Emergency Nursing Nursing Tips

Peripheral Nerve Testing Peripheral Nerve Hand Therapy Peripheral Nerve Injury

A Week In The Woods By Andrew Clements Scholastic Com Andrew Clements Kids Book Club Book Club Books

Favorite Lunch Spots In Nashville Tn Hattie B S Hot Chicken The Pharmacy Two Boots Pizza Rippy S Bar G Banana Pudding Hot Chicken Banana Pudding Recipes

12 Hour Shift Funny Nurse Quotes Nurse Quotes Night Shift Humor